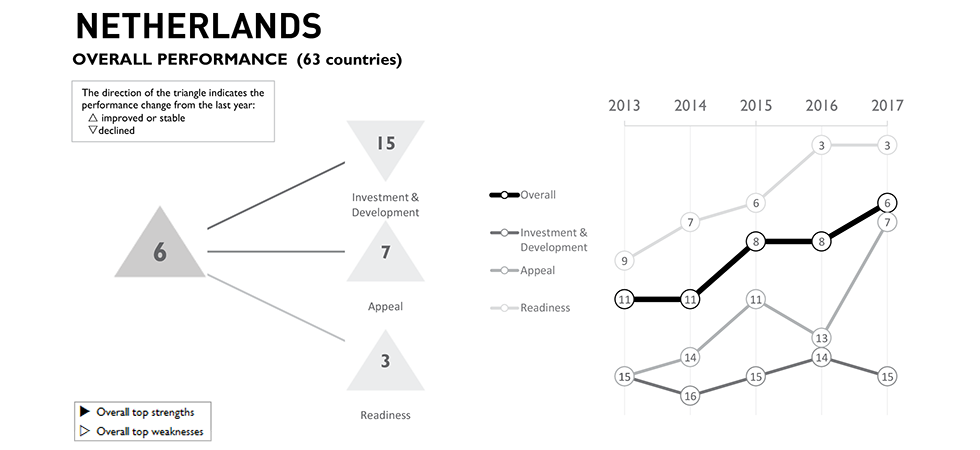
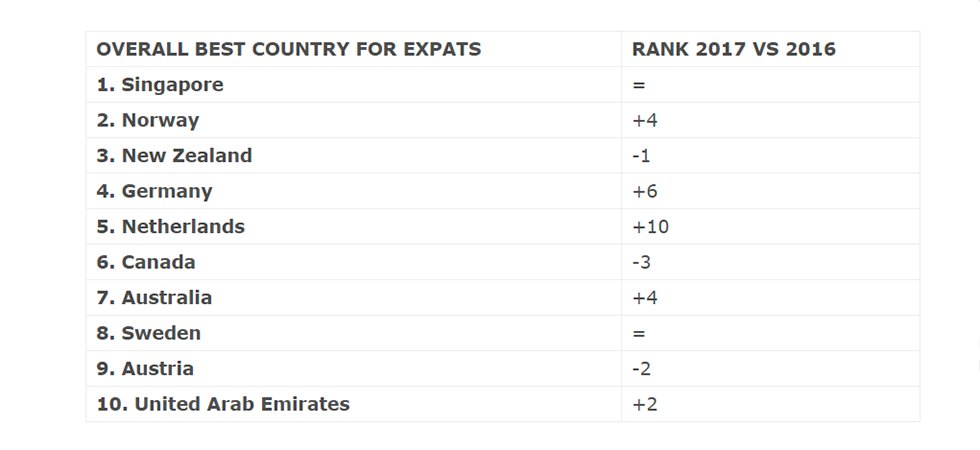
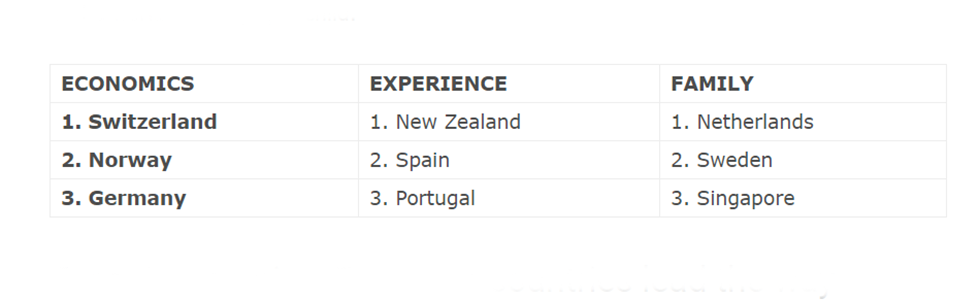
Scale-ups in the Netherlands
The Scale-up Dashboard 2017 shows that:
- The number of scale-ups in The Netherlands has increased to 3237. This means that the number of scale-ups increased by 5.4% in the past year compared to the previous year.
- The number of startups becoming scale-ups rises tremendously. Over the past two years, this has increased by no less than 220%. One in ten scale-ups in the Netherlands arise from a startup.
- Top sectors count relatively many scale-ups. The top sectors Energy, High-tech and Life Sciences & Health are at the frontrunners.
- An increasing number of companies in the Netherlands are hardly, if at all, growing. Almost a third of Dutch companies are even shrinking.
More startups become scale-ups
Prof.dr. Justin Jansen: “The Scale-up Dashboard is the first list that truly takes into account and measures all companies in the Netherlands with more than 10 FTE. There are more lists about scale-ups available, but those give limited insights because companies have to register themselves or the lists are focused on specific sectors. Insight in which companies truly belong to the Top of scale-ups is missing and that is what we hope to achieve by creating and launching this Top 250 Scale-ups (Top 250 Groeibedrijven) – of over 3000 scale-ups – in the Netherlands.”
“Although more and more startups are making the step to scale-up, considerable efforts are still needed to keep countries like China, Israel and America up and running”, says Prince Constantijn van Oranje, special envoy of StartupDelta. Find out what he has to say more about the current position of startups and scale-ups in the Netherlands and the research findings (interview BNR in Dutch).
Interested? Have a look at the preview above or request the Scale-up Dashboard 2017 via www.ece.nl. A similar dashboard (in Dutch) is available for scale-ups in Zuid-Holland.
Source / ECE